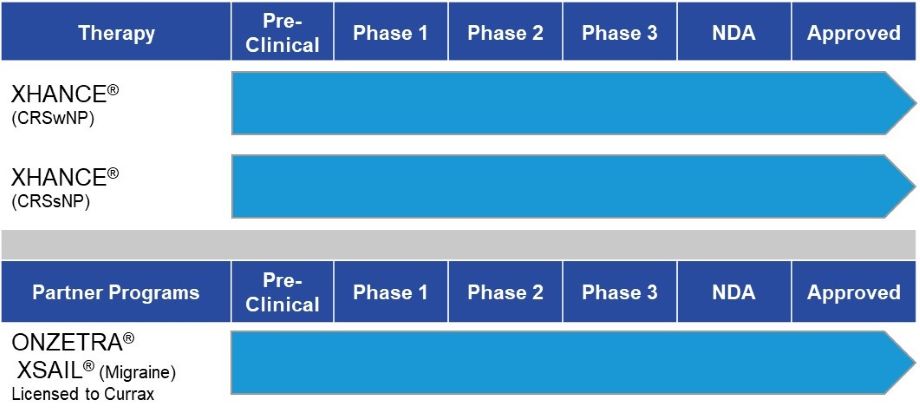
Research & Development
Other Drug-Device Product Candidates Combining Drug with Exhalation Delivery Systems
Although our current focus is to prioritize the successful commercialization of XHANCE for the ENT and allergy specialty segment, we may apply or out-license our Exhalation Delivery System (EDS) technology to other product candidates across a broad range of disease areas. For example, by placing drug high and deep in the nose, in regions where cranial nerves connect directly with the brain, we believe it may be possible to deliver medications directly into the brain and avoid the difficulties of getting drug past the blood-brain barrier. This may enable treatment of brain diseases using small or large molecules that otherwise do not readily enter the nervous system. Other potential examples where deep and broad intranasal delivery may create differentiated clinical benefits include delivery of vaccines to antigen-presenting immune cells throughout the upper respiratory tract to boost humoral (IgG), mucosal (IgA), and cell-mediated immune responses, rapid introduction of drugs to the blood to enhance speed of onset, and treatment of local conditions related to the nose and related structures (e.g., the olfactory cleft, trigeminal ganglion, etc.).
For questions, please contact us at medical.services@optinose.com.
